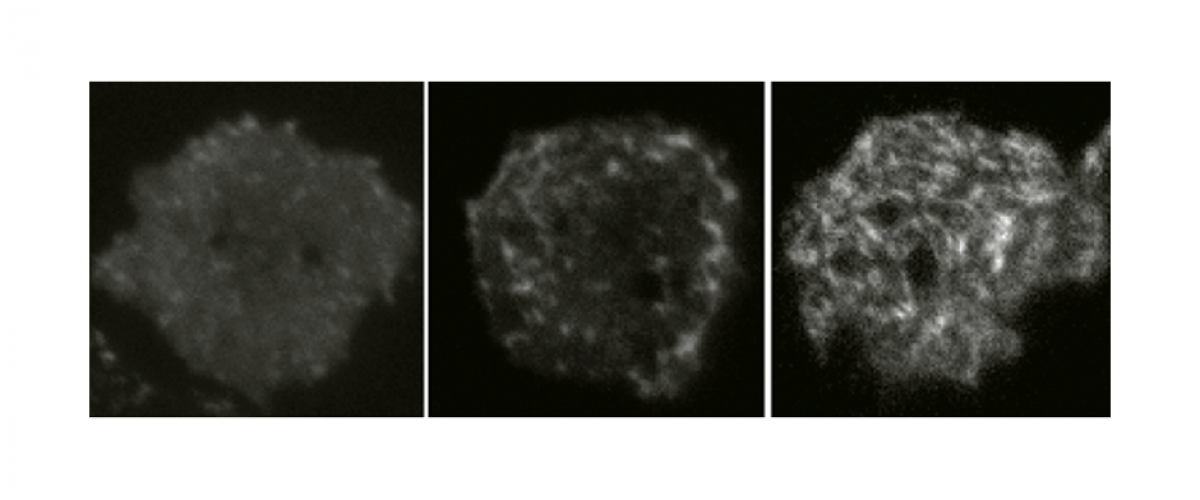
While much is understood about how myosin II is regulated by light chain and heavy chain phosphorylation, we still do not know how the protein is specifically localized. Mechanosensing through cooperative binding of myosin motors to allosteric actin filaments is one major mechanism. To identify new mechanisms, we performed genetic suppression of a phosphomimic (3xAsp) myosin II, which poorly assembles into bipolar thick filaments, and found several genetic suppressors that rescued assembly and/or cleavage furrow accumulation. Several new proteins were identified, including the Regulator of Microtubule Dynamics-1 and methylmalonate semialdehyde dehydrogenase. For more information, please see Ren et al. 2014.